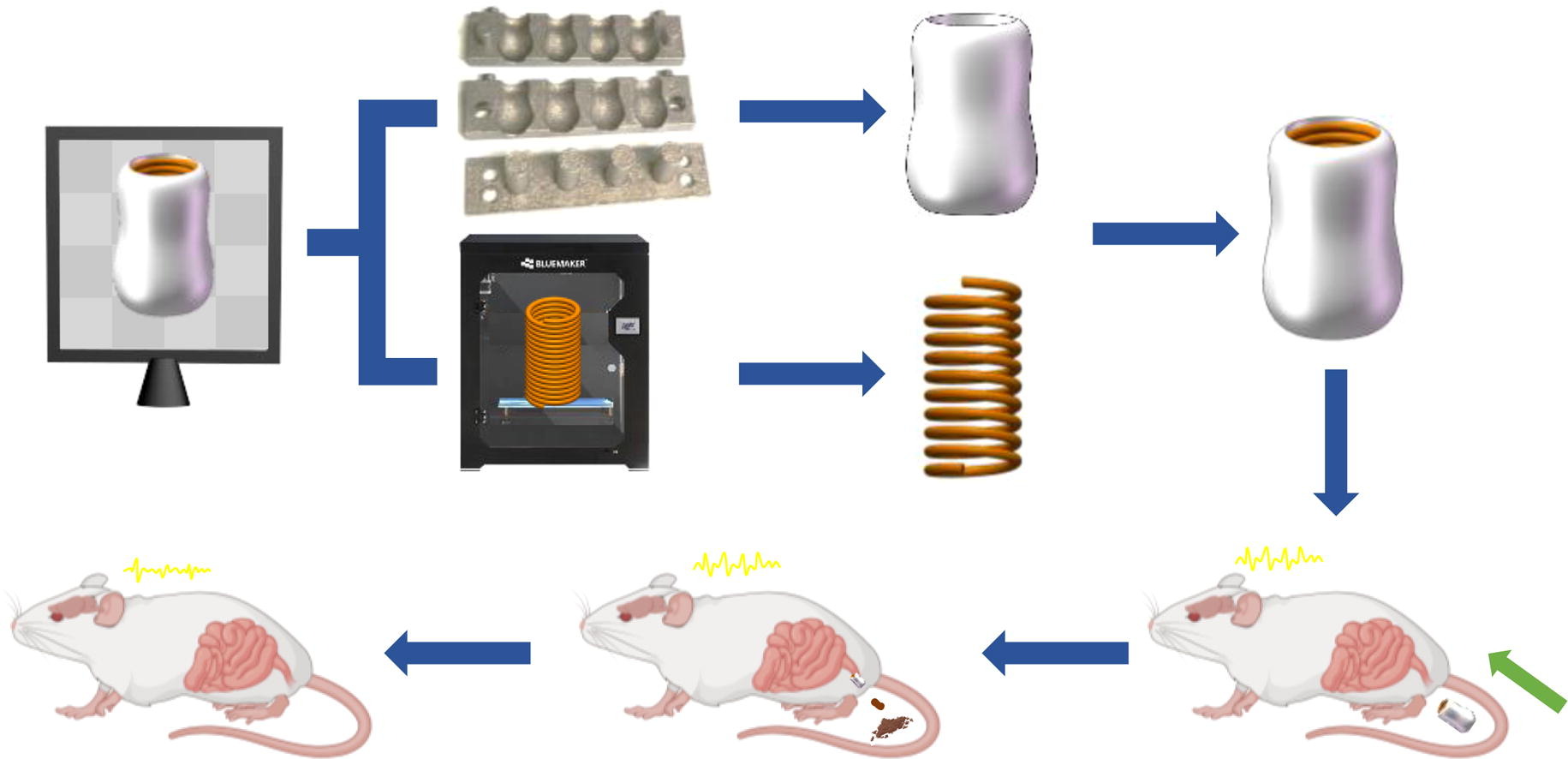
Researchers from the Beijing Institute of Radiation Medicine have found that cannabidiol (CBD)-loaded hollow suppositories using 3D printing technology may be effective in treating epilepsy.
3D-printed CBD suppository.
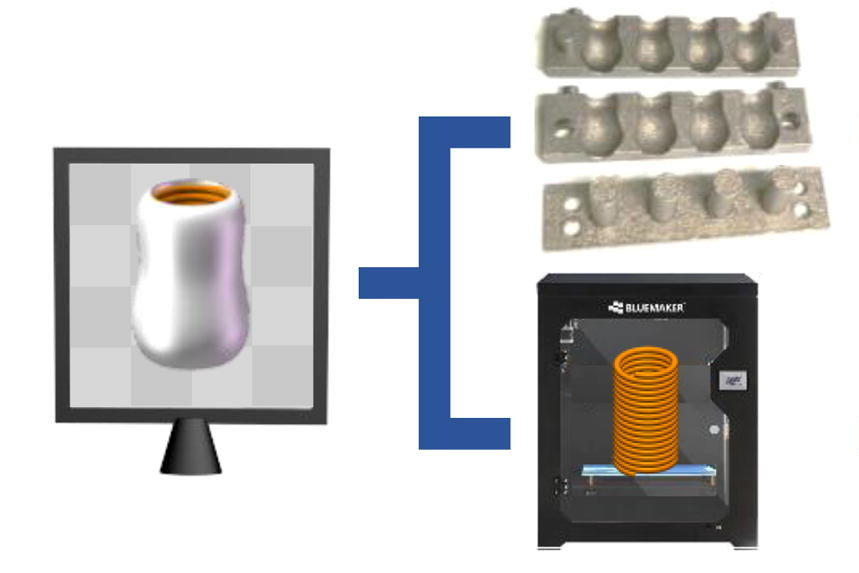
Published in the International Journal of Pharmaceutics, the study highlights the limitations of traditional oral CBD formulations, which often face significant first-pass metabolic effects, reducing their efficacy. The newly designed suppository, referred to as CHS (cannabidiol-loaded hollow suppository), features an inner supporting spring and an outer CBD-loaded hollow shell.
The spring was created using 3D-printed thermoplastic urethane filaments, while the shell was crafted with a metal mold filled with a mixture of CBD, polyvinyl alcohol, and polyethylene glycol. Laboratory tests showed that CHS provided a sustained release of CBD over five hours, allowing effective systemic delivery.
Tests on epilepsy-induced rat models revealed significant benefits. Locally administered CHS alleviated brain damage and reduced inflammation. Furthermore, the treatment improved gut microbiota composition, increasing beneficial bacteria such as Lachnoclostridium and Akkermansia.
The study’s full abstract can be found below, and its full text can be found by clicking here.
Abstract
Cannabidiol (CBD) is widely used to alleviate the syndromes of epilepsy. However, the marketed oral CBD formulation has the prominent first-pass effect. Here, a cannabidiol-loaded hollow suppository (CHS) was developed using three-dimensional (3D) printing technology. CHS was assembled with an inner supporting spring and an outer CBD-loaded curved hollow shell. The spring was prepared using fused deposition modeling 3D printing with thermoplastic urethane filaments followed by splitting. The shell was prepared with a 3D-printed metal mold filled with the mixture of CBD, polyvinyl alcohol, and polyethylene glycol. CHS slowly in vitro released CBD for 5 h and achieved the systemic delivery of CBD. The high in vitro and in vivo safety of CHS was demonstrated. Epilepsy rat models were established by lithium-pilocarpine dosing. Locally administered CHS greatly alleviated the damage to brains and reduced inflammation. Moreover, CBD obviously improved the abundance and composition of gut microbiota and the abundance of beneficial bacteria, including Lachnoclostridium and Akkermansia. Personalized CHS is a promising medication for the treatment of epilepsy.